Pierre
Curie
(1859-1906)
Marie Curie (1867-1934)
Born in Warsaw : Maria Sklodowska
Working in a labs like this, Marie and Pierre worked to investigate the
properties
of uranium bearing rock and the other radioactive elements it contained.
The term and concept of 'radioactivity' was created and developed by
Marie.
Pierre and his students experimented with alpha, beta, and gamma 'rays'.
Marie discovered and named the elements Radium and Polonium.
The Curies and Antoine Becquerel
won the 1903 Nobel Prize in Physics
for their investigations of radioactivity.
Marie won a 1911 Nobel Prize in Chemistry
for discovering Radium, Polonium and many of their chemical properties.
The health dangers of radioactive substances were not well known ...
and Marie died of cancer.
The dangers of horse-drawn vehicles were well known ... but Pierre was
crushed under one anyway.
... it just goes to show you ...
Much of the information in the 1908 textbook mentioned below came
from research they had conducted.
Contemplating 'The Atomic Age'
Throughout the course of history, philosophers, alchemists, and
scientists
speculated about, and experimented to discover, the smallest pieces of
matter.
However, the dawn of 'The
Atomic
Age' is associated with the Manhattan Project in America ...
because it sounds very manly to say that in an echo chamber when
writing one's own publicity and history.
The human ability to throw the nuclei of heavy, unstable elements into
uncontrolled,
explosive, nucleus-fissioning chain reactions is what resulted from the
Manhattan Project.
Generally, the human 'control' of these forces was limited to ...
* The air-portability of the bomb.
* Creating an explosion of a predictable size.
* The ability to start an uncontrolled chain reaction on cue.
This is exactly what this $2
billion enterprise was intended to achieve.
What happened next ... and why
it happened ...
is what pages 2 and 3 in this series try to explore.
|
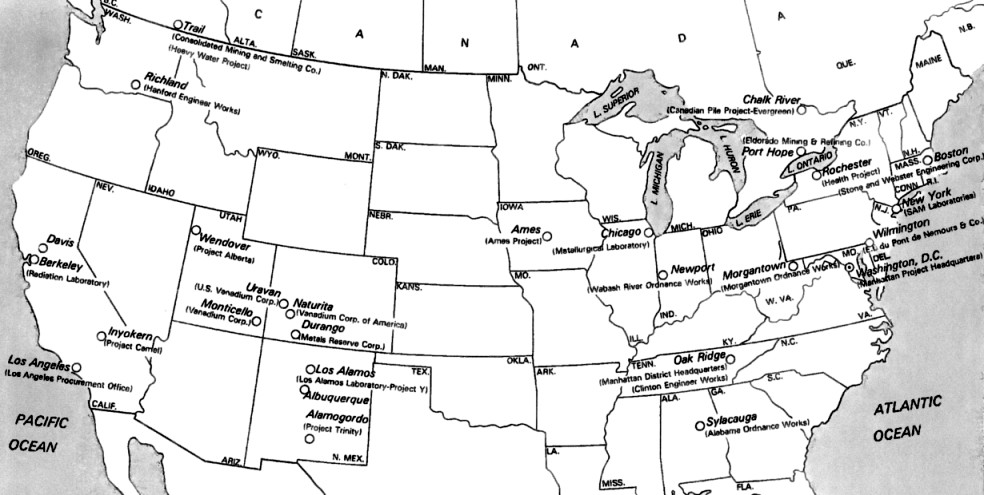
Radioactive Substances in 1908
As the
British textbook 'The Radioactive
Substances' from 1908
shows,
considerable work had already been done to understand the different
radioactive elements.
The text states how the different forms of the radioactive elements
named are obtained or refined by chemical treatment. The table below
shows the half-lives of selected isotopes before the current notation scheme
was developed ... which adds the atomic mass to the elemental symbol.
'Emanation' refers to a gas
coming from a sample after a specific chemical treatment.
Today we know that the alpha 'ray'
is actually a
particle ... which has the two proton/two neutron structure of a helium
atom ... and which carries a 2+ charge. It is good at ionizing
(stripping
electrons) but does not penetrate thin physical barriers ... such as a
grocery list written on graph paper. Ionizing 'radiation' is damaging
to living tissue in
general ... and can cause cancer and genetic damage.
Today we know that the beta 'ray' is
a
high speed,
high energy ... electron (1- charge) ... [or a positron (1+ charge)]
... It is a
medium ionizer with medium penetrating ability ... it probably couldn't
fight its way out of an unopened can of spruce beer.
Only the gamma rays
are true electromagnetic radiation. Today,
their characteristics are essentially defined the same as the
characteristics X-Rays. Lead
and thick concrete barriers are necessary to protect living tissue from
them.
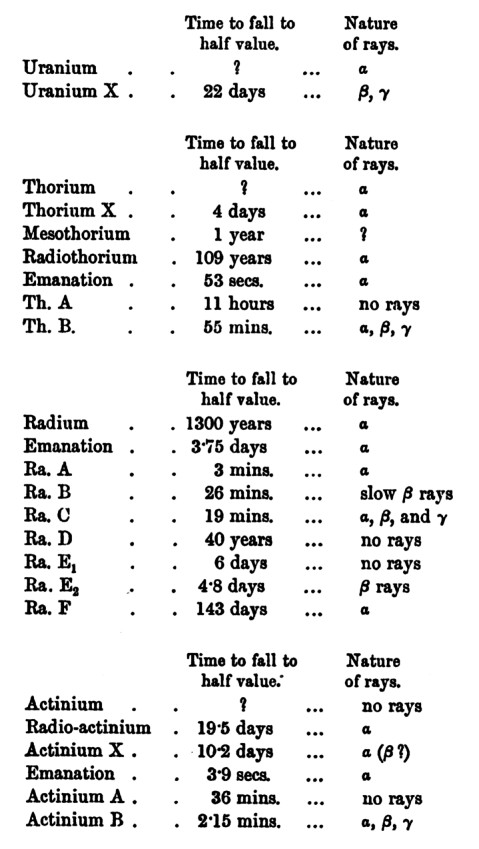
Below,
circa 1900, exploring the characteristics of the various 'rays'.
The alpha and beta 'rays' have a charge ... as they change direction in
the magnetic field.
Speculation: If these two are particles and not 'rays' ...
the stronger deflection of the beta suggests its mass is less than the
alpha's.
This work was done by Ernest
Rutherford (1871-1937)
He named the alpha and beta 'radiation' in 1899.
He devised the catchy name for gamma radiation in 1903.
GAM-MA. Cool.
In 1906 his experiments determined that the alphas were in fact
7000 times heavier than the beta particles.
Back in 1902, Rutherford and Soddy had theorized that radiation was the
result of spontaneous disintegration of the atoms of some elements
... and in the process, they believed ... one element was converted into other
elements.
When he discovered that the alpha particles coming from radium were actually helium atoms ... it pretty much
supported their theory.
You could probably do a whole web page on this guy all by himself !

Einstein in his
early 20s.
Einstein was born in 1879 in today's 'southern Germany'. Although he
spent time working and teaching in universities in other European
countries, including
Germany, it was in Switzerland in 1905 that he published his most
famous and insightful ideas ...
- Light acts as packets of energy ... not just as a wave as
had been observed.
- Particle theory ... the random movement of
small articles supports atomic theory ... think of skunk aroma
molecules - they go everywhere!
- Special Relativity ... the ending to '2001 A Space Odyssey'
... or was that General
Relativity ... ?! ... uh ... no ... General Relativity came later ...
- Mass and energy are linked - not separate 'things'. The E = mc2
equation
represents the idea that a small mass of matter contains a lot
of energy ... c, the speed of
light, is a big number, eh? A large proportion of this energy could be
liberated by
changing the composition of the atom's nucleus.
Most of our common chemical
reactions work 'far above' the nucleus in the electron zone
of various atoms ... and the molecules of which they are part.
High explosives are perhaps the most dramatic example of these
'electron level' reactions.
But to try an experiment at the atomic
level in your home ...
Get some atoms and line them up
on a table ... until they form a line which is one inch in length.
Count them, and you'll find 1,000,000,000,000 of them in your inch-long
arrangement.
If you had a really good pair of tweezers and could hold on to a SINGLE
atom ...
And could split the nucleus of this SINGLE atom by firing neutrons at
it ...
and could watch closely with your naked eyes ...
it would release enough energy to make a little grain of
sand hop.
* * *
Good old Newtonian physics from the late 1600s was still good
enough
for
constructing buildings, designing airplanes, and building optical
telescopes and microscopes - every day useful stuff on planet Earth.
Our earthbound mammalian brains and civilizations evolved perceiving ... only
electromagnetic radiation with a wavelength of 400-700 nanometres ...
i.e.
'light' ... moving in straight lines [OK we do perceive 'heat'
too, but to mention that would make this paragraph very long-winded ...
are you happy now?] ... and we habitually perceived objects no larger than
oceans
or mountains ... and objects no smaller than little specks of dust
floating
in the air ... and we evolved experiencing a little awe for any living
thing or any object
which had existed for more than 100 years ... often with the
help of an antique dealer.
One thing which makes Einstein seem freaky and awe-inspiring,
himself, to most humans
... is that he worked with the knowledge obtained from scientific
experiments ...
about very large, very small, and very old things ... very fast things ... and phenomena
of very short
duration ... things beyond the realm of our normal human experiences.
Examples ... the speed of light ... the size and span of the
universe ... the smallest fragments
of matter/energy ... how light and 'gravity' interact in space.
Regarding the last phenomenon,
Einstein predicted that a
star's light would be bent by the sun's gravity.
May 1919, Sobral, Brazil;
and later in 1919 at Principe off the coast of today's Gabon :
during solar eclipses, what Einstein had only predicted ... was
observed for the first time.
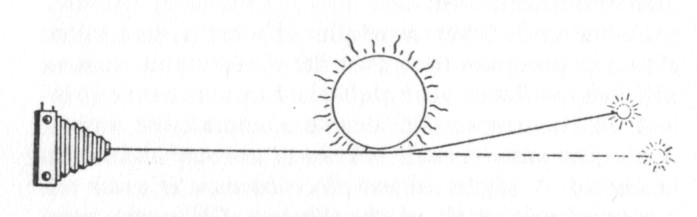 Astronomers (and ship navigators back then) knew where most stars
were in the sky at any given time.
Astronomers (and ship navigators back then) knew where most stars
were in the sky at any given time.
With the sun's disk and the whole sky darkened by the eclipse ... stars
could be seen by day.
Although a given star was expected to be 'behind' the sun (solid line)
...
it would be visible as if it were actually at the dashed line location
...
proving Einstein's 'Relativity' calculations which looked at light and
gravity in a new way.
... in a few words ... Einstein sometimes
summarized his conclusions like this ...
"Time and space are indivisible from the moving body and must be
regarded relative to that movement. In this respect time and space are
relative ... In place of the old metaphysical conception of pure time
and space having only geometrical qualities, we obtain a new theory of
time and space inseparable, bound up with bodies and movement." ... summary by Academician M.
Mitin, Soviet philosopher (1942)
Einstein was not an experimental
physicist. He did not spent a lot of time grinding up rocks, putting
them in acid and/or or a vacuum, bombarding them with ionized
particles or looking through telescopes.
Einstein was a theoretical
physicist.
He did a lot of day-dreaming with thought experiments and came up with
new ideas to explain the universe. He
also plugged the facts and numerical values that were known into
elegant math equations to support these new ideas. He believed
the simplest, most 'symmetrical'
explanation supporting what he observed ... was best.
His 1921 Nobel prize was mainly for the 'light is particles' work (the
particles later named 'photons') ... and apparently not for the famous E = mc2
equation and energy/matter relationships which 40 years later yielded atomic bombs
and nuclear power.
... but in his native Germany some
physicists just couldn't debate professionally - even with Einstein ...
"Jewish physics can best and most justly be characterized by recalling
the activity of one who is probably its most prominent representative,
the pure-blooded Jew Albert Einstein. His relativity theory was to
transform and dominate all physics; but when faced with reality, it no
longer has a leg to stand on. Nor was it intended to be true. In
contrast to the equally intractable and solicitous desire for truth of
the Aryan scientist, the Jew lacks to a striking degree any
comprehension of truth - that is, anything more than an apparent
agreement with a reality that occurs independently of human thought."
Philipp
Lenard - born in today's Bratislava.
Nobel Prize winner 1905 for research on cathode rays.
Chief of Aryan Physics
under Hitler.
Quote from his book German Physics.
In Lenard's 1938 book Great Men of Science
(66 men),
he starts with Pythagoras, and later includes da Vinci, Galileo,
Pascal and Newton ...
later still, it is on to ...
Watt, Coulomb, Volta, Ampere, Ohm, Joule,
Kelvin, Hertz ...
he included Darwin, too ...
but no mention of Einstein ...

The Fuhrer's own scholars
and Kultur police ... at one of many Fahrenheit 451 Book Barrel Bashes.
Fuhrer's Funfact: Einstein journal articles, bound in hardcover, burn
longer!
The next author suggests that Philipp
Lenard and his friends were just too ...
practical ...
and too Newtonian ...
"The characterization of the Einsteinian definitions of 'length',
'temporal duration', and so on, as 'lifeless' in contrast to the
definitions of traditional physics has only the following
justification. At every stage of scientific development concepts are
introduced by means of such definitions as correspond to the particular
stage; that is, they are as practical as possible for the presentation
of available knowledge. When such a stage has lasted for a long time,
the words that are used in science gradually become words of daily
life; they acquire an emotional overtone and become filled with life.
Every introduction of new definitions appears to us to create
'lifeless' concepts.
"I once met on a train a Japanese diplomat who was just coming from the
Wagner festival at Bayreuth. I asked him how he liked Wagnerian music.
He replied: 'Technically it is highly developed and ingenious. But in
comparison with Japanese music it lacks a soul.' For one who has grown
up with the sound of Japanese music in his ears Wagnerian music sounds
just as 'lifeless' and 'intellectualistic' as the definitions of
Einstein's theory do to one who has been accustomed all his life to
Newtonian mechanics."
Philipp
Frank, Austrian physicist and philosopher,
nominated by Einstein as his replacement at Prague.
Quote from his book Einstein, His Life and
Times (1957)
* * *

Einstein and some relatives on
the move somewhere between Germany and America in 1930.
In Germany, Einstein had abandoned
a nice waterfront house which he had recently purchased ...
and he was not wealthy.
Having just left Germany so it could benefit from its pure 'Aryan
Physics'...
and keeping his Swiss citizenship safely in his back pocket ...
he went to the German embassy in Brussels, Belgium and renounced his
German citizenship.
Well ... there was no way to do this !
vunce a churman ...
Fortunately, Germany efficiently set up a new process by which they
could revoke the citizenship of undesirables.
"As Einstein allows his world-famous name to be used as the cover for
lying propaganda ..."
Sources suggest he never returned to Germany.
From 'Uranium and Atomic Power' (1941) ...
again ... before the
Manhattan Project
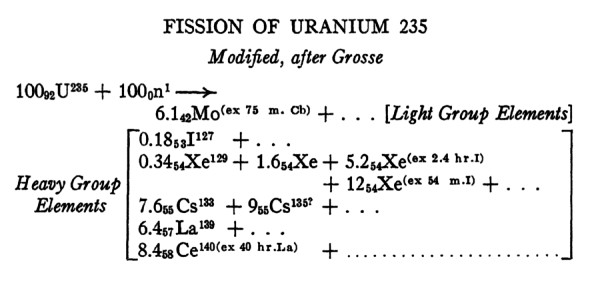 Today's nuclear waste ...
Today's nuclear waste ...
Problem # 1: You get many different radioactive products.
Problem # 2 appears on the next page in this series.
This 1941 book, published in the
US,
gives a comprehensive description of Uranium - prospecting, physics,
chemistry, its potential for atomic energy and how to measure its
activity. It was this type of generally available knowledge which
galvanized the scientists in Chicago, and elsewhere, to seek Einstein's
help (in the next section below).
As you can see ... combining a neutron with U-235 was known to give
a
wide variety of fission products in various quantities ... in this 1941
understanding of the phenomenon. As you can imagine, this is one of the
problems with today's
nuclear generating plant waste. It is not possible to 'wear out' or
'burn up' the
radioactivity of a given quantity of nuclear 'fuel' to make it safe.
There is a good chance that one or more radioactive elements ... with half-lives longer than recorded
human civilization ... will be mixed all through the 'waste'.
With a run-away atomic reaction ... such as that which you would want
for an atomic explosive ... things happen very fast within the
'explosive charge'. Extensive calculations and experiments would be
necessary to
ensure that MOST of the U-235 (for example) nuclei are in position to
be bombarded efficiently with chain reaction neutrons in the
microseconds BEFORE the whole mass of U-235 starts to physically break
up.
... Otherwise the explosive charge will 'fizzle' with a small explosion
and precious, unfissioned U-235 all over the place.
Much of Einstein's early genius had passed by 1939 ...
but he was still good to have on your side.
After 1905, it was people like
Rutherford and Fermi and Bohr, Hahn, Strassmann, Meitner, Frisch,
Szilard, and Teller who further developed the ideas surrounding
'splitting the atom' and releasing all that energy from tiny bits of
matter.
Preferring the certainty of myths and pseudo-science ... rather than
understanding the ideas of 'Jewish' scientists like Einstein ...
Hitler, Mussolini,
and colleagues caused many
bright scientists to flee continental Europe. In
1933, with Hitler's ascension to the office of German Chancellor, it
was clear that things
in and near Germany were going to get rather unpleasant. The European
emigrant scientists found willing research partners
in their new homes in Britain and America.
The weight of Einstein's name - he was now living in the
US - was needed to get the attention of US President Roosevelt. This
was done
through a letter written together by Szilard and Einstein. The
emigres were concerned that Hitler's outfit might design an atomic
weapon. The letter referred to below was sent at the beginning of
August 1939 - a month
before Hitler invaded Poland, the latter event beginning World War 2.
In later life
Einstein was a faculty member at the
Institute for Advanced Study at Princeton, New Jersey
... from 1933 until his death in 1955.
POLITICS
Key passages from the letter sent from Szilard and Einstein to
Roosevelt ...
" Some recent work by E. Fermi and
L. Szilard, which has been communicated to me in manuscript, leads me
to expect that the element uranium may be turned into a new and
important source of energy in the immediate future. Certain aspects of
the situation which has arisen seem to call for watchfulness and, if
necessary, quick action on the part of the Administration.
" I believe therefore that it is my duty to bring to your attention the
following facts and recommendations:
" ... it has been made probable by the work of ... that it may become
possible to set up a nuclear chain reaction in a large mass of uranium,
by which vast amounts of power and large quantities of new radium-like
elements would be generated. Now it appears almost certain that this
could be achieved in the immediate future.
" This new phenomenon would also lead to the construction of bombs ...
extremely powerful bombs of a new type may thus be constructed ... A
single bomb of this type, carried by boat and exploded in a port, might
very well destroy the whole port together with some of the surrounding
territory.
" The United States has only very poor ores of uranium in moderate
quantities. There is some good ore in Canada and the former
Czechoslovakia, while the most important source of uranium is Belgian
Congo.
" ... you may think it desirable to have some permanent contact
maintained between the Administration and the group of physicists
working on chain reactions in America ... "
The letter recommended
that a person might be designated ...
" ... to approach Government Departments, keep them informed ...
to put forward recommendations for Government action, giving particular
attention to the problem of securing a supply of uranium ore for the
United States
" ... to speed up experimental work ...
" I understand that Germany has actually stopped the sale of uranium
from the Czechoslovakian mines which she has taken over. That she
should have taken such early action might perhaps be understood on the
ground that the son of the German Under-Secretary of State, von
Weizsacker, is attached to the Kaiser-Wilhelm-Institut in Berlin where
some of the American work on uranium is now being repeated. "
Watching the numerous
documentaries, movies, and TV
mini-series made about the Manhattan
Project ... you generally see a thoughtful, and later morally-tormented
J.Robert 'Oppie' Oppenheimer
(1904-1967). He was
the scientific director of the Manhattan Project and he is often
portrayed with a little team of
brilliant but temperamental foreign scientists with foreign accents ...
working in the desert at the former boys'
school which had become the Los Alamos Laboratory compound. The
dramatic action
of most modern re-enactments was portrayed here at Los Alamos ... where
the very first uranium and plutonium bombs were
designed and built.
 Physicists: Paul Dirac,
Robert Millikan, J. Robert Oppenheimer
in the 1930s.
Physicists: Paul Dirac,
Robert Millikan, J. Robert Oppenheimer
in the 1930s.

Enrico
Fermi
|
* Neutrons Neutrons Neutrons
The whole field of nuclear chain reactions
begins with
getting enough neutrons ...
hitting enough fissionable nuclei ...
with just the right energy.
Unlike protons with their positive charge, a neutron
can slip - relatively easily - into a nucleus and make it unstable.
The unstable nucleus
'splits' and releases a lot of energy in the process. But just firing
neutrons and getting atoms to split doesn't usually get you very much.
As discussed above, you are only releasing enough energy from each
nucleus to
make a grain of sand hop.
The design of your pile must have
a large enough mass of nuclei
('critical' mass) ... so the neutrons
released from atom splitting ... hit enough additional nuclei to sustain
a chain reaction ... that is, without your help, the pile is continually throwing off more
neutrons.
Cadmium, graphite
(the special form of carbon used in pencil 'leads'), heavy water, etc.
are used because of their effect on neutrons.
Sometimes
these
substances are used to regulate the neutrons so they travel
at 'your speed limit'. At other times you want to mop neutrons up ...
so the chain reaction cools down ... or expressed another way - so the
internal generation of chain reaction neutrons effectively stops.
In a working bomb, obviously, you have no plans to stop the chain
reaction.
You also need really PURE materials. Even slight
impurities in good quality industrial graphite were found to mop up too
many precious neutrons before they could
do anything towards chain-reacting.
|
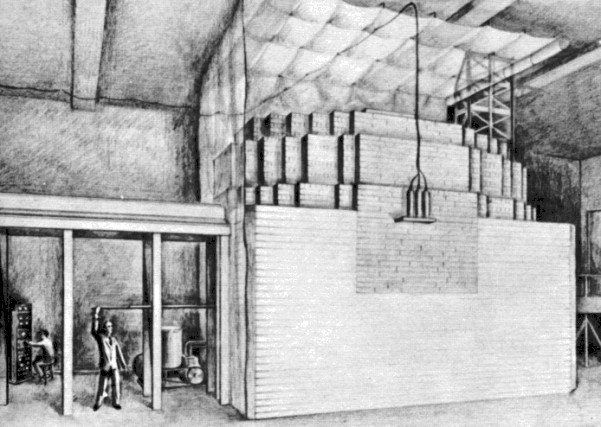 The first controlled chain reaction of nuclear fission happened in
Chicago, December 2, 1942.
The first controlled chain reaction of nuclear fission happened in
Chicago, December 2, 1942.
On that occasion, there were significantly more participants and
observers present.
As the pile went 'critical' - i.e. a self-sustaining chain
reaction -
a backup safeguard was a man with an axe.
In the event that the reaction ran away ...
Fermi would instruct the axeman to cut the rope which suspended
additional emergency cadmium rods.
These rods would drop back into the core area, and would
absorb enough neutrons to stop the reaction ...
so this part of the University of Chicago would not travel skywards.
Another key character portrayed (sometimes by Brian Dennehy or Paul
Newman) is Brigadier General Leslie
Groves (1896-1970).
He is correctly depicted as the intelligent and hard-driving military
boss of the whole project ... if you want something done fast and done
right during a war - give it to the right Army officer ... an Army
officer believes that 'his' mission to develop a powerful new bomb will
save soldiers' lives. Groves was a very capable
'systems' guy who got stuff done.
 After the Trinity Test of the
plutonium bomb at Alamogordo ...
Groves is crunching his way across the
sand turned into radioactive glass at Ground Zero.
At the right is a scientist with
Geiger counters.
The bomb was exploded at the top of a
steel tower to minimize fallout.
Except for the four bases shown, the
tower was vapourized.
After the Trinity Test of the
plutonium bomb at Alamogordo ...
Groves is crunching his way across the
sand turned into radioactive glass at Ground Zero.
At the right is a scientist with
Geiger counters.
The bomb was exploded at the top of a
steel tower to minimize fallout.
Except for the four bases shown, the
tower was vapourized.
A
very complex project ... the 129,000 'extras' you never see ...
In a television or motion picture drama about the Manhattan Project ...
it is difficult to portray the
massive effort in industrial
engineering and construction; research;
human resources management and security; planning and process
sequencing ... to
complete the atomic bomb project.
At its peak in June 1944, 129,000
workers were employed in
construction and operations, with 100,000 people remaining on the
project into 1945.
The project had raw materials coming from the Belgian Congo or northern
Canada
... and later ... dangerous, delicate, very scarce and precious end
products
to be delivered to a yet-to-be-selected-and-built ... high-security
air base on some Pacific Island in a war zone. Japanese submarines were
actively
watching for, and sinking, US warships at the most promising locations.
In between Africa & Port
Radium in Canada ... and Tinian Island, these 'extras' simply worked at
these locations ...
There were numerous contracts made
with many universities and scientists. Some activities were located
in very isolated areas on purpose, some were located where there was
abundant electrical power, others where particular labs or pieces of
industrial
equipment were available.
Some massive facilities bred plutonium, other large operations
separated U-235 from the much more
common U-238. Finally, when more than a few milligrams or grams were
available for study, the scientists could then determine which material
might be feasible for a bomb.
Seeing tons and tons of ore and raw materials enter their facilities,
and the same volume of tailings going out the back door ...
and never seeing the mere grams of radio-active materials shipped away
under guard to Los Alamos or Tinian ... some workers concluded they
were providing an industrial diversion
and actually producing nothing
... to confuse the enemy.
Think of
this single question ... as an example of one of the thousands of
problems to be
solved ...
As different isotopes of
uranium are the same element,
you
couldn't use a chemical
process to separate them, because the two isotopes would react
similarly ... Even if you HAD pure (highly corrosive) uranium metal,
how
might you separate atoms of something which was about 3 atomic mass
units lighter (U-235) than most of your supply (U-238) ? Theoretical
possibilities ...
- Ionize your uranium, and accelerate it as a
beam with magnets. Then swing this beam of ionized uranium around a 180
degree curve with the same magnetic force. The
U-238 will take the corner too wide. Capture the U-235 ions after they
make their tighter turn.
- U-235
is lighter and will bounce
around more. Get a membrane with millions of pores per square inch and
allow your gaseous uranium atoms to bounce around. Then take
whatever went through your membrane : U-235 and U-238 ... and repeat
this many,
many
times through many identical subsequent stages. You will eventually get more and
more U-235 on the far side of these membranes. This is described as a
'statistical method' ... so be patient.
 1. These are some of the magnet
elements (lying down) used for electromagnetic separation of U-235 from
U-238.
1. These are some of the magnet
elements (lying down) used for electromagnetic separation of U-235 from
U-238.
 2. This is the size of facility
required for the 'statistical' method
of gaseous diffusion to separate U-235
from U-238.
2. This is the size of facility
required for the 'statistical' method
of gaseous diffusion to separate U-235
from U-238.
The Test at Alamogordo
The Plutonium Implosion Bomb: Test
Trinity
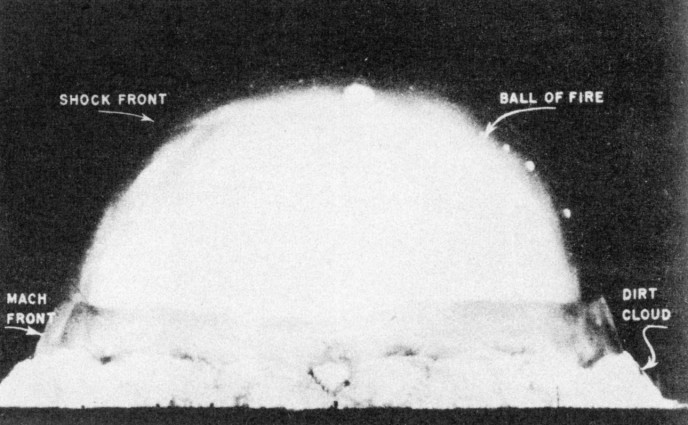
"If the radiance of a thousand
suns were to burst at once into the sky, that would be like the
splendor of the mighty one."
"Now I am become Death, the destroyer of worlds."
Before the bombs were dropped ...
This publication was prepared for public release
at the request of General Groves:
It contains about 150 pages of
dense fact-filled reading. The idea was to include ...
"All
pertinent scientific information which can be released to the public
at this time without violating the needs of national security is
contained in this volume. No requests for additional information should
be made to private persons or organizations associated directly or
indirectly with the project. Persons disclosing or securing additional
information by any means whatsoever without authorization are subject
to severe penalties under the Espionage Act.
"The success of the development is due
to the many thousands of scientists, engineers, workmen and
administrators - both civilian and military - whose prolonged labor,
silent perseverance, and whole-hearted cooperation have made possible
the unprecedented technical accomplishments here described."
L.R.
Groves, Major General, War Department, Washington, August 1945
* * *
"...
It is neither a documented
official history nor a technical treatise for experts. Secrecy
requirements have affected both the detailed content and general
emphasis so that many interesting developments have been omitted.
"References to British and Canadian
work are not intended to be complete since this is written from the
point of view of the activities in this country.
" ... The average citizen cannot be
expected to understand clearly how an atomic bomb is constructed or how
it works but there is in this country a substantial group of engineers
and scientific men who can understand such things and who can explain
the potentialities of atomic bombs to their fellow citizens."
H.D.
Smyth [author], July 1, 1945